Vietnam Launching Covid-19 Nanocovax Vaccine Clinical Trial .Vietnam currently has four vaccine manufacturers and research agencies working on a jab against novel coronavirus disease (COVID-19), offering favorable conditions to have a made-in-Vietnam vaccine ready by the end of 2021, a health official said.

Nguyen Ngo Quang, deputy director of the Administration of Science, Technology and Training under the Ministry of Health, made the remarks at a Hanoi meeting on research, production, clinical trials, licensing and the use of vaccines in Vietnam on Wednesday.
Speaking at the event, a representative of the international, nonprofit global health organization PATH said that there were 163 COVID-19 candidate vaccines in different stages of development around the world as of July 15.
Among them, 23 are in the clinical testing phase while 140, including those from Vietnam, are in the preclinical stage.
In Vietnam, there are four companies and institutes, namely VABIOTECH, Polyvac, IVAC, and Nanogen, working on a COVID-19 vaccine and they have gained positive initial results.
Among them, the research between IVAC, the Icahn School of Medicine at Mount Sinai in New York, Thailand’s Government Pharmaceutical Organization (GPO), and the Brazilian vaccine producer Instituto Butantan are promising high feasibility, according to Quang.
IVAC’s candidate vaccine was sent to the U.S. for testing on animals on July 20.
If everything goes as planned, the vaccine will be ready for human clinical trials in October this year, the institute’s director Duong Huu Thai said.
Meanwhile, VABIOTECH, or the Company for Vaccine and Biological Production No.1, is also working with the University of Bristol in the UK on a possible COVID-19 shot.
VABIOTECH conducted preclinical research trials on animals in May, which produced satisfactory results and good immune response.
It is expected that the company will test its COVID-19 vaccine on humans by the beginning of 2021.
Mr. Nguyen Ngo Quang, Deputy Director of Department of Science, Technology and Training, Ministry of Health, said that the first phase of clinical trials will evaluate safety and initially assess the immunogenicity of the vaccine. The Ministry of Health has asked the research team to develop a protocol using a dose of 25 mcg to ensure absolute safety for participants in the phase one trial. They must evaluate the safety of this trial dose and then continue to inject the next subjects, increasing the dose to 50 and 75 mcg.
At the end of phase one, researchers will evaluate which is the safest injection dose to move to phase two. Phase two will use the results of phase one research to deploy, at the same time, vaccinate children under 12 years old or elderly over 60 years old.
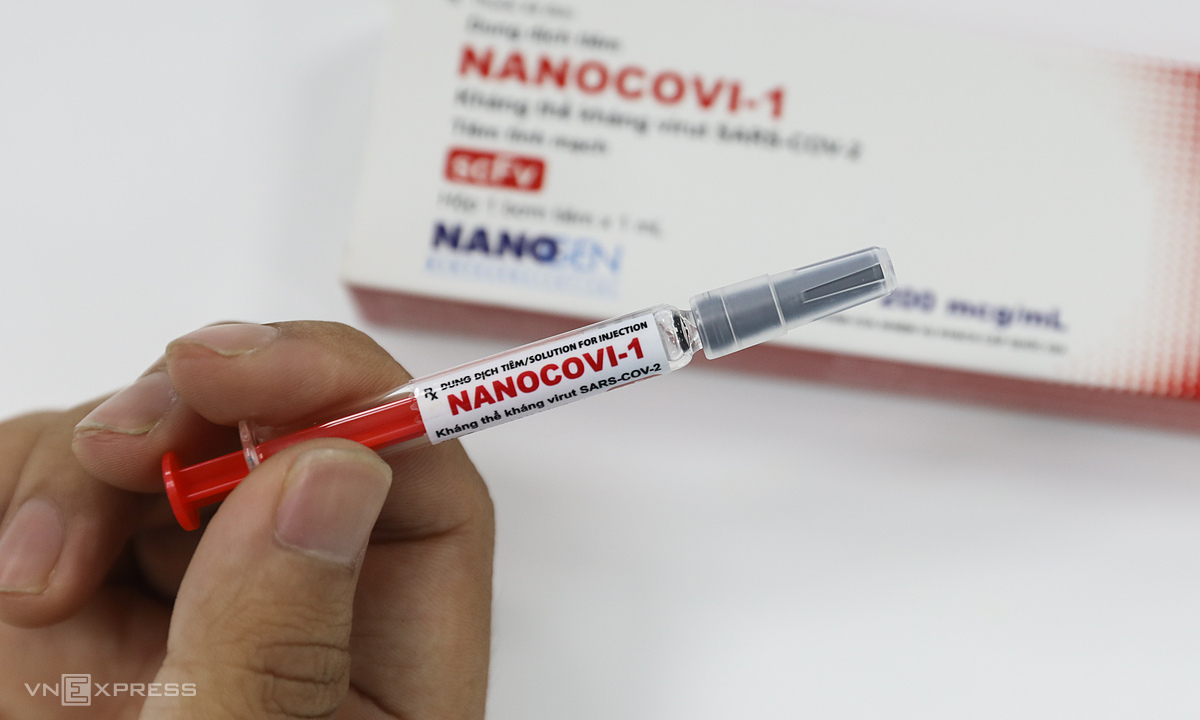
Covid-19 vaccine named Nanocovax is researched and produced by Nanogen Company. Photo: Quynh Tran.
According to Mr. Ho Nhan, General Director of Nanogen Company, Nanocovax research unit, the company’s clinical trial results on mice, monkeys, and rabbits at the National Institute of Hygiene and Epidemiology are good. After vaccinating mice for 1-2 weeks, the company gave two groups of rats that were vaccinated and not vaccinated with nCoV.
“The vaccinated rats are still normal, gaining weight, eating normally, while mice not vaccinated have clear signs of illness. When being tested, mice that were vaccinated gave negative results, mice that were not vaccinated gave positive results” he said.
If the human clinical trial is also good, Nanogen plans to complete the trial by May 2021, consult the Prime Minister and the Ministry of Health for an urgent license. In the short term, vaccines will be administered to groups frequently exposed to epidemics, at high risk such as medical staff, flight attendants and the elderly, with underlying medical conditions.
Nanocovax is the first Covid-19 vaccine in Vietnam in a clinical trial in humans. Vietnam currently has 3 more Covid vaccines in the process of animal testing.